
Chemistry |
Aldehyde compounds,
extracted from oak,
capable of crosslinking tannins and
anthocyanins
|
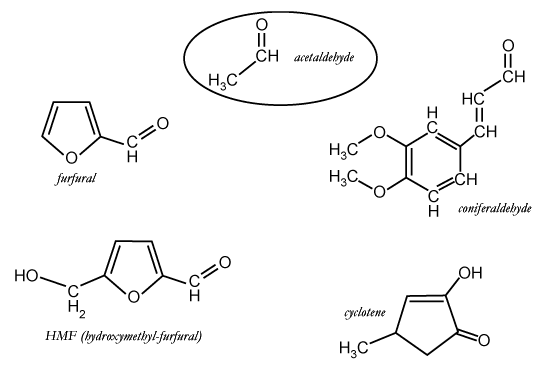
Sugar
dehydration products, formed during toasting of oak, e.g. furfural and
HMF (shown above), are able to crosslink tannins and tannins with anthocyanins.
However, Cheynier's group has shown the aromatic aldehydes, e.g. vanillan and
coniferaldehyde (shown above), have not been found to crosslink tannins.
Evidently, there is a steric hindrance by the aromatic group. However, the same
aromatic group may allow hydrophobic interaction with tannins and anthocyanins,
allowing the aromatic aldehyde to act as a co-factor in
co-pigmentation.
|
Crosslinking procyanidins with aldehydes |
There are
clear structural differences between crosslinked procyanidins and natively
formed (or reductive linked) procyanidins. The important point here is that
different procyanidin or tannin structures can be formed when acetaldehyde or
oak extractives are present.
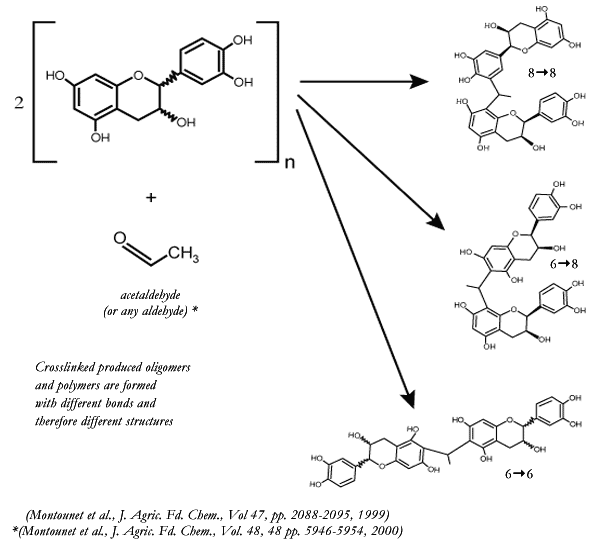
|
Tannin-anthocyanin (T-A) /tannin-tannin (T-T)
crosslinking under oxidative conditions |
|
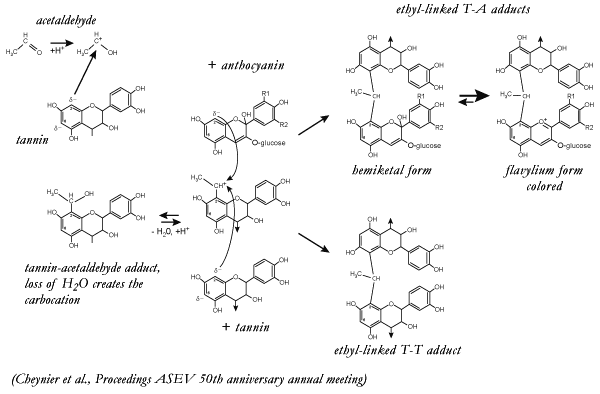
The proposed mechanism of
crosslinking between procyanidins and anthocyanidins
follows:
Acetaldehyde has been shown to react first and
preferentially with the procyanidin or tannin, not with anthocyanins. However,
as Cheynier's group established, the crosslinker could be furfural, HMF and
other oak derived components. Secondly, acetaldehyde tannin adduct with
dehydrate to form a carbocation. From this point, the reaction is much the same
as described previously for reductively formed adducts. Depending on
concentrations and proximity for reaction, the carbocation had the choice of
reacting with either tannin or anthocyanin. As before, the anthocyanin reacts
in the hydrated or colorless form and the tannin acts as the electro-sink to
favor elimination of water, thereby forming the flavylium cation and
stabilizing the color. |
Proposed condensation reactions under oxidative
conditions |
|
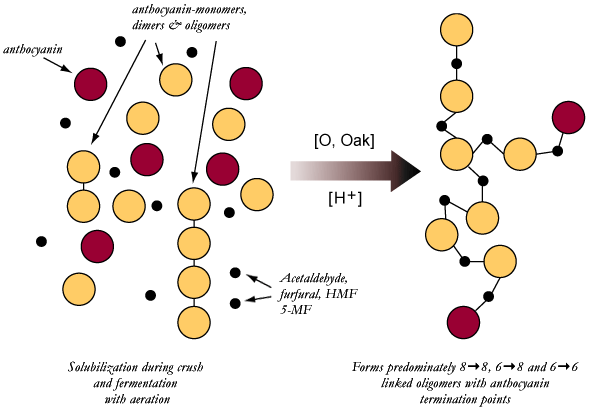
Above is
a simplified pictorial of the crosslinking reaction. The main point of interest
is the difference in the structure comparing crosslinked vs. reductively formed
or native tannin oligomers. The many kinks added into the oligomer or polymer
prevent tight association and hence aggregation minimizing
precipitation.
The crosslink formed between the procyanidins is
no more stable than those existing in the native procyanidin. Therefore, they
are labile and prone to hydrolysis, just as negative oligomers and polymers
are. However, Cheynier's group has found that crosslinks formed with furfural,
HMF and 5-methyl furfural are more stable (less prone to hydrolysis) than those
made with acetaldehyde. Remember that linking an anthocyanin to a tannin
prevents further polymerization at that end. We should see a higher percentage
of smaller oligomers formed when acetaldehyde or oak derived crosslinkers are
present. This should be especially prevalent during fermentation, when
anthocyanins are in high concentration and available for
reaction.
|
Acetaldehyde-induced condensation of
epicatechin and malvidin-3-glucoside
|
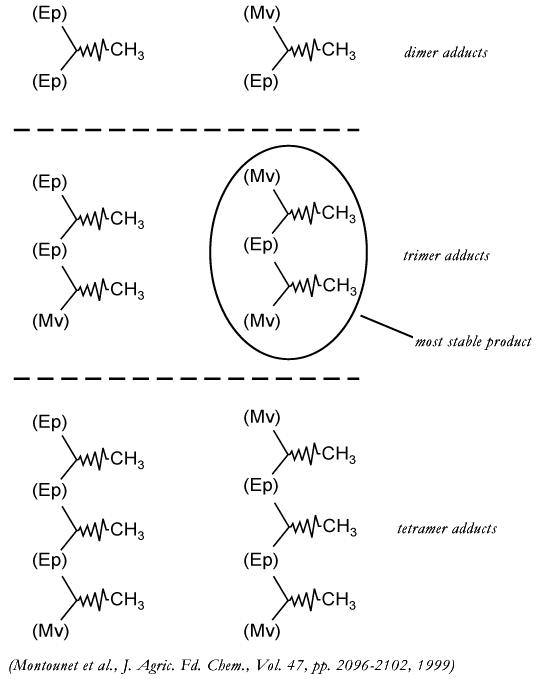
In model solutions, we should see more
smaller polymers formed when enough reactants (procyanidins and anthocyanins)
are available. The trimer adduct should be the most stable that is
formed.
|
|
|
|







