
Chemistry |
General anthocyanin
structure
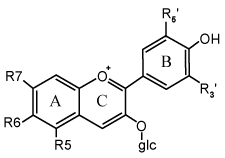 The second major class of
compounds found in red wines are anthocyanins. They have a positive charge on
the molecule which enables it to absorb light and thus have color. An
anthocyanin has a carbohydrate (sugar, usually glucose) esterified at the 3
position. An anthocyanidin, termed the aglycone, does not have a sugar at the 3
position. Naturally occurring pigments from grapes always have a sugar bonded
at the 3 position, though other compounds such as hydroxycinnamates and acetate
may be involved. The presence of this sugar helps the anthocyanin maintain
solubility in water. If the sugar is hydrolyzed or lost, the solubility
decreases and the molecule will be destabilized and lost.
The second major class of
compounds found in red wines are anthocyanins. They have a positive charge on
the molecule which enables it to absorb light and thus have color. An
anthocyanin has a carbohydrate (sugar, usually glucose) esterified at the 3
position. An anthocyanidin, termed the aglycone, does not have a sugar at the 3
position. Naturally occurring pigments from grapes always have a sugar bonded
at the 3 position, though other compounds such as hydroxycinnamates and acetate
may be involved. The presence of this sugar helps the anthocyanin maintain
solubility in water. If the sugar is hydrolyzed or lost, the solubility
decreases and the molecule will be destabilized and lost.
|
| Major anthocyanin forms occurring at wine
pH |
|
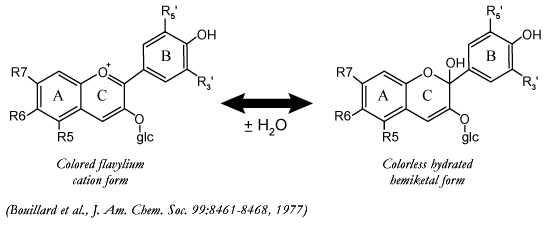
All
naturally occurring anthocyanins are in equilibrium between the color flavylium
cation and the colorless hydrated form. The equilibrium is driven to the left
as the pH of the wine is decreased and to the right as pH is increased. At pH's
above 4.5 other destabilizing reactions begin, such as ring opening of the C
ring.
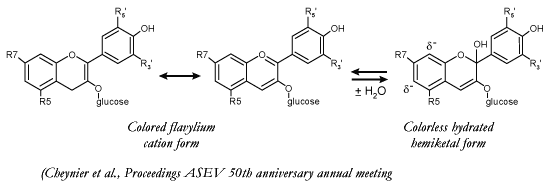
Why is the hydrated or colorless
form of the anthocyanin important? Véronique Cheynier has proposed that
the hydrated form is actually the reactive form of the anthocyanin. When water
hydrates the anthocyanin, it adds 2 electrons to the molecule, which in turn
increases the electro-negativity at the 6 and 8 positions on the A ring.
Generally, it appears that the 8 position is the favored postion of
reaction. |
|
|
|







